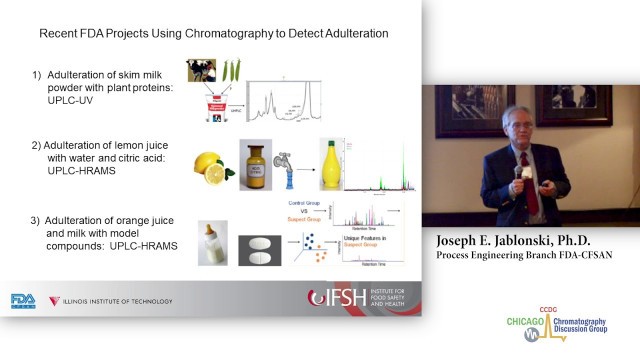

'Presenter: Joe Jablonski, PhD Economically-motivated adulteration (EMA) of food continues to be a problem and a potential health danger. The melamine adulteration of milk which was discovered in 2008 is a well-known example. Analytical chemistry is used to authenticate food products, and to measure adulterant chemicals and/or cheaper ingredients which cause economic loss or physical harm. Examples of food adulteration detection methods developed in FDA laboratories will be presented. Historical examples, and recent work with milk powder and fruit juice employing modern chromatographic methods will be discussed. Current methods which use high-resolution chromatography and mass spectrometry generate very large data sets. This is particularly the case for so-called \'non-targeted\' methods which evaluate the entire set of components and features in sample data files. Advanced data processing techniques are needed to optimize the quality of the data identify adulterated samples with a high degree of confidence. Dr. Jablonski is a research analytical chemist in the Process Engineering Branch of FDA-CFSAN, he works at the Moffett Center in Bedford Park, IL. He received his B.Sc. degree in biology from University of Notre Dame and PhD. in chemistry from Ohio University. He has extensive experience in chromatography and mass spectrometry related to analysis of environmental, pharmaceutical and food samples. The first half of his career was spent in industry at Monsanto Agricultural Products in Missouri, and then at Ricerca Biosciences in Ohio. He has been conducting research on chemical contaminants and adulterants in food matrices at FDA since 2003. Dr. Jablonski\'s early work at FDA centered on analysis of acrylamide in fried foods. His current research projects involve assessment of nano-composite packaging materials, and heavy metal contamination of beverages from diatomaceous filter aids.'
Tags: FDA , food adulteration , chromatography , economically-motivated adulteration , melamine in milk , FDA Laboratories , CCDG , FDA-CFSAN , Chromatographic methods
See also:






!['Top 5 Best Stand Mixers of [2021]'](https://cdn-img01.wefoodblog.com/images/48-m/138/1387029_m.jpg)









comments